Listen to this article
Gene therapy for SMA, particularly with the groundbreaking treatment Zolgensma, is revolutionizing the way we approach spinal muscular atrophy, a condition that has historically posed severe challenges for affected families. This innovative therapy offers a glimmer of hope, enabling patients like young Edward to achieve remarkable milestones that were once deemed impossible. With advancements in gene therapy making strides in SMA treatment, Edward, who received Zolgensma as a baby, is now able to walk independently, showcasing the transformative potential of this one-time therapy. As more children receive such interventions, the expectations for SMA progress are changing, painting a brighter future for those diagnosed with this severe genetic disorder. The ongoing development of gene therapies like Zolgensma initiates a new era in the fight against SMA, promising a quality of life improvement for many children affected by this condition.
In recent years, significant strides in genetic interventions have opened new pathways for tackling challenges associated with spinal muscular atrophy, or SMA. The emergence of therapies such as Zolgensma has shifted the landscape of SMA management, providing affected children with remarkable opportunities for growth and development. By utilizing cutting-edge gene therapy, families are witnessing firsthand the potential for life-altering benefits that these treatments can offer. With more children experiencing advancements in their health, the conversation around SMA and available treatment options is gaining momentum. As awareness and understanding of these transformative therapies spread, the hope for innovative solutions to combat severe genetic disorders continues to grow.
The Breakthrough of Gene Therapy in Treating SMA
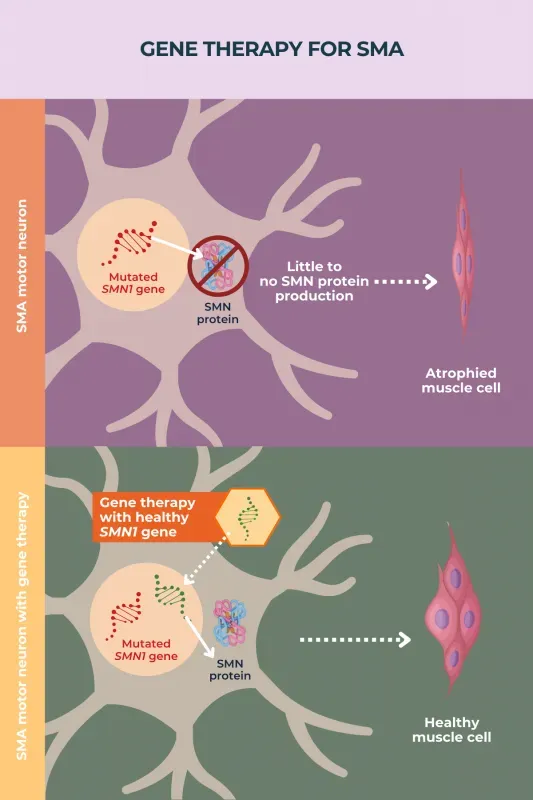
Gene therapy has revolutionized the treatment landscape for spinal muscular atrophy (SMA), a debilitating genetic disorder characterized by muscle weakness and atrophy. One of the most significant advancements in this field is the introduction of Zolgensma, a one-time gene therapy that targets the root cause of SMA by providing a copy of the missing or defective SMN1 gene. This innovative treatment not only improves motor function in children affected by the condition but also enhances their overall quality of life. With SMA primarily affecting infants and young children, gene therapy offers hope for families as it addresses issues related to muscle development that traditional treatments, such as Spinraza, cannot resolve with the same efficacy.
In recent years, we have witnessed remarkable progress in how gene therapy is applied for SMA, allowing patients to undertake activities that were once thought impossible. For example, Edward, a young boy from England, was one of the first patients to receive Zolgensma. His inspiring journey illustrates the substantial impact that gene therapy can have, enabling him to walk independently and engage in ordinary childhood activities. As medical advancements in gene therapy continue, we expect further breakthroughs, which will not only push the boundaries of existing treatments but also pave the way for potential cures for other genetic disorders.
The impact of gene therapy for SMA goes beyond just physical improvements; it also fosters emotional growth and social inclusion among affected children. With treatments like Zolgensma, patients are not merely surviving but thriving, exhibiting milestones associated with typical development. Families, like that of Edward, experience a transformation in their hopes and aspirations for the future. Edward’s mother, Megan, expressed her amazement at his progress, reflecting on how he evolved from a lethargic infant to an energetic child full of personality. Such profound changes challenge the previously bleak outlook associated with SMA, which often confines children to a lifetime of dependency and health struggles without intervention.
Furthermore, as more children receive Zolgensma and other gene therapies, researchers and healthcare professionals are gathering invaluable data to evaluate the long-term effects of these treatments. Observing patients as they age may redefine expectations for children with SMA, potentially allowing them to lead lives that resemble those of their healthy peers. This shift signifies a new era for SMA treatment, where advancements in gene therapy could ultimately see this condition transform from a tragic diagnosis to a manageable one.
Understanding Zolgensma: The Cost and Controversies
Zolgensma, a groundbreaking gene therapy, has generated significant attention not only for its efficacy but also for its staggering price tag of £1.79 million. Debates surrounding the cost of Zolgensma highlight critical discussions in the medical community about accessibility and affordability. For many families, like that of Edward, gaining access to this revolutionary treatment depended on negotiations with healthcare systems or personal fundraising efforts. Edward’s mother, Megan, initiated a fundraising campaign that successfully raised £170,000 to support his medical needs and therapies, emphasizing the financial burden that many families face while seeking lifesaving treatments for their children.
While NHS England negotiated undisclosed discounts on Zolgensma’s original price, the discussion surrounding the ethics of such high prices for potentially lifesaving treatments remains ongoing. Advocates argue that the investment in gene therapy could ultimately lead to significant savings in long-term healthcare costs as healthier patients require fewer medical interventions over time. As the conversation about the economics of rare disease treatments evolves, it underscores the necessity for structured healthcare policies that can ensure broad and equitable access to cutting-edge therapies like Zolgensma.
The controversy surrounding the pricing of Zolgensma also extends to the medical community’s perceptions of its effectiveness and long-term viability. Being relatively new, long-term outcomes research on Zolgensma is still in its early phases, leading to a mix of optimism and caution among advocates and skeptics alike. As Professor James Palmer of NHS England noted, Edward’s transformative experience after receiving treatment supports the potential of gene therapies to significantly change lives and provides a glimpse into a future where other devastating genetic disorders might be treatable as well.
As more success stories emerge, pulses of excitement ripple through the SMA community, fueling hopes that this generation of children may indeed grow up to lead normal, fulfilling lives. The road to wider acceptance of pricing and treatment protocols will undoubtedly continue to be challenged, but the necessity of weighing immediate costs against long-term benefits cannot be overstated. It is this balance that will ultimately drive the conversation forward, influencing regulatory guidelines and healthcare policies designed around innovative treatments for rare diseases.
The Role of Physiotherapy in Supporting SMA Progress
While gene therapy like Zolgensma marks a significant leap forward, its effectiveness is highly complemented by ongoing physiotherapy. For children with spinal muscular atrophy, regular physical therapy sessions can greatly enhance the recovery and development of muscle strength and coordination, which are crucial for independence and mobility. Edward’s experience highlights this, as he undergoes physiotherapy up to five times a week to build upon the improvements gained through gene therapy. His mother’s dedication to securing specialized care exemplifies the essential role that physiotherapy plays in the overall treatment plan for SMA.
Physiotherapy helps in preventing complications that arise from the muscular atrophy associated with SMA, fostering mobility, and providing a foundation for functional skills. In Edward’s case, his remarkable ability to learn swimming and regain strength after a double hip replacement showcases the transformative potential of combining gene therapy with regular physiotherapy. This multi-faceted approach not only cultivates physical strength but also instills confidence in the children as they pursue everyday activities, reinforcing their sense of normalcy and integration within their communities.
Moreover, physiotherapy can also have profound psychological benefits for children with SMA and their families. The process of rehabilitation fosters personal growth and allows children to set achievable goals, which can significantly enhance their self-esteem. After starting school and making friends, Edward has been able to engage in typical activities, something that would have seemed unattainable before his treatment. His mother expresses pride in his achievements, underscoring the emotional lift that progress in physical therapy can afford both the child and family.
As more families embark on similar journeys, the need for comprehensive plans that encompass gene therapy, ongoing physiotherapy, and community support becomes apparent. Insisting on integrated care models ensures families are not just participants in a treatment journey but are empowered advocates for their children’s futures. With advancements in both gene therapy and supportive physiotherapy, we anticipate a future where children with SMA can thrive and reach their fullest potential.
The Future of SMA Treatment and Research
The advent of gene therapy, particularly through treatments like Zolgensma, is paving the way for a new frontier in SMA research and treatment. As ongoing studies and clinical trials explore the long-term effects and broader applications of gene therapy, the possibility of transforming the prognosis for SMA continues to gain traction. With each success story, like Edward’s, the medical community is motivated to explore innovative treatment options that could potentially extend beyond SMA to other genetic disorders, showcasing the potential of genetic intervention in modern medicine.
Moreover, advancements in genetic research are giving rise to a reimagined understanding of how such therapies could operate across varied conditions, expanding the horizon for what might once have seemed insurmountable. Researchers are optimistic that the same techniques that have benefited SMA patients could eventually lead to breakthroughs in treating other complex genetic disorders, beckoning a new era of personalized medicine.
As treatment protocols evolve, the importance of educating families about available options remains paramount. Increased awareness can empower parents to advocate for their children effectively and navigate the complexities of healthcare systems. Communities, nonprofits, and advocacy groups play crucial roles in disseminating information about gene therapy developments, as well as fundraising initiatives aimed at increasing accessibility to such treatments.
Looking to the future, we can anticipate a landscape wherein SMA and similar conditions are met with informed support, robust treatment options, and a continually growing body of research that aims to unlock the mysteries of genetic disorders. As we witness today, many children like Edward may prove to be the trailblazers of an unprecedented paradigm where children with SMA can achieve remarkable milestones, inspiring hope for future generations.
Frequently Asked Questions
What is gene therapy for SMA and how does Zolgensma work?
Gene therapy for spinal muscular atrophy (SMA) aims to replace the missing or malfunctioning SMN1 gene, critical for producing a protein necessary for muscle function. Zolgensma is a one-time gene therapy that delivers a functional copy of the human SMN gene to motor neuron cells, leading to improved muscle strength and function, which can dramatically enhance the quality of life for those affected by SMA.
What are the advancements in gene therapy for SMA, specifically regarding Zolgensma?
Advancements in gene therapy for SMA, particularly with Zolgensma, represent a significant breakthrough in treatment options. Zolgensma is unique as it provides a one-time intervention that can lead to lasting effects, transforming the lives of children by promoting muscle development and strength. Clinical outcomes show that many children who received this therapy have made remarkable progress and can achieve milestones they previously could not.
How effective is Zolgensma in treating spinal muscular atrophy?
Zolgensma has proven to be highly effective in treating spinal muscular atrophy (SMA), with reports of children making ‘incredible progress’ post-treatment. Patients like Edward have shown significant gains in mobility and overall development, achieving milestones typical for their age, which underscores the therapy’s potential to alter the course of the disease.
What are the potential long-term benefits of receiving Zolgensma for SMA patients?
The potential long-term benefits of receiving Zolgensma for SMA patients include improved motor function, the ability to perform everyday activities independently, and an overall enhanced quality of life. Early treatment has shown promising results, with optimistic expectations that children treated with this gene therapy could reach adulthood, a feat previously unimagined for many with SMA.
Why is Zolgensma considered the most expensive drug in the world?
Zolgensma is considered the most expensive drug in the world primarily due to its groundbreaking technology and the complexities involved in its development and administration. The one-time treatment costs £1.79 million, which reflects the extensive research and innovations in gene therapy for spinal muscular atrophy (SMA) that have brought forth life-changing treatments.
What are the alternative treatments available for SMA besides Zolgensma?
Besides Zolgensma, alternative treatments for spinal muscular atrophy include Spinraza, which requires ongoing spinal injections to increase SMN protein levels, and other emerging therapies that are currently in clinical trials. Each treatment has unique benefits and considerations, making it essential for caregivers and patients to discuss options with healthcare providers for optimal care.
How can families access Zolgensma for their children diagnosed with SMA?
Families can access Zolgensma for children diagnosed with spinal muscular atrophy (SMA) through healthcare systems, such as the NHS, which may cover the cost of the treatment if criteria are met. Additionally, fundraising initiatives play a crucial role for some families, allowing them to access private care and therapies while awaiting approvals or additional treatments.
What role does physiotherapy play in the recovery of children treated with gene therapy for SMA?
Physiotherapy plays a vital role in the recovery of children treated with gene therapy for spinal muscular atrophy (SMA). Following treatments like Zolgensma, regular physiotherapy can help children improve muscle strength, develop motor skills, and achieve greater independence. It supports the overall effectiveness of the gene therapy and enhances the gains made after treatment.
| Key Point | Details |
|---|---|
| Patient Background | Edward is a five-year-old boy from Colchester diagnosed with spinal muscular atrophy (SMA). He is one of the first patients in England to receive gene therapy. |
| Gene Therapy Administered | He received Zolgensma, the world’s most expensive drug, costing £1.79 million, through the NHS in 2021. |
| Progress Since Treatment | Edward has shown ‘incredible progress’ including walking independently and engaging in activities typical for a five-year-old. |
| Milestones Achieved | He can swim, jump off boats, and has started school, where he makes friends and participates like other children. |
| Ongoing Care Needs | Edward may require a wheelchair for life, but his mother emphasizes the importance of his happiness over physical limitations. |
| Family’s Financial Challenges | Megan initiated a fundraising campaign for Edward’s ongoing care, raising £170,000 for private physiotherapy and equipment. |
| Future Outlook | Despite uncertain long-term outcomes, there is optimism that future generations of SMA patients might have improved quality of life due to gene therapy. |
Summary
Gene therapy for SMA is revolutionizing the treatment landscape for patients like Edward, who has made significant progress since receiving Zolgensma. This innovative therapy addresses the root cause of spinal muscular atrophy by delivering a functional copy of the SMN1 gene. The success story of Edward not only highlights the potential of gene therapy but also raises awareness about the challenges and financial burdens families face. As research continues, there is hope that more advancements will pave the way for improved therapies for SMA and similar genetic disorders.