Listen to this article
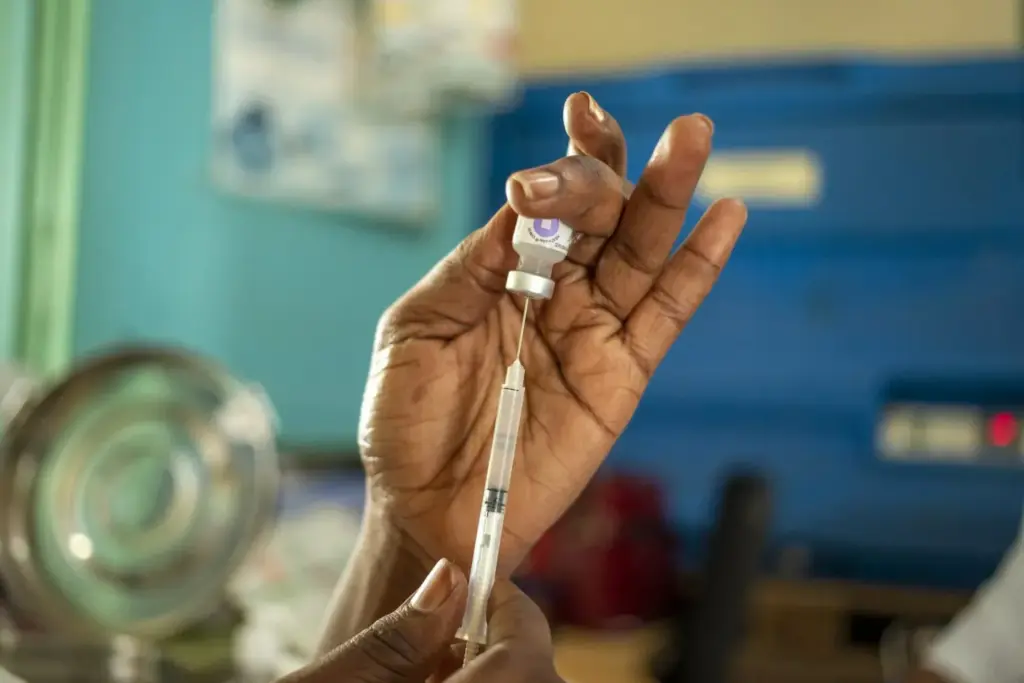
The Guinea-Bissau vaccine trial, a controversial US-funded study focused on the hepatitis B vaccine, has recently drawn sharp criticism from the World Health Organization. This proposed trial intended to explore the effects of administering the vaccine to newborns at birth versus at six weeks, sparking ethical concerns about the implications for infant health. With hepatitis B being a significant public health issue in Guinea-Bissau, the WHO emphasized the importance of the birth-dose vaccine as a lifesaving intervention. They argue that withholding the vaccine from some infants in the trial could lead to irreversible harm, challenging the very foundations of ethical medical practices. As discussions continue, the trial raises questions about vaccine ethics and public health interventions in vulnerable populations, highlighting the ongoing debate around responsible vaccine implementation globally.
The exploration of vaccine research within Guinea-Bissau has recently sparked heated discussions, particularly surrounding a planned study on the hepatitis B immunization in infants. Dubbed as a pivotal investigation, this trial aimed to assess timing and effectiveness of vaccine administration among newborns, igniting widespread ethical concerns from global health authorities. Many view this as a crucial moment for understanding the implications of newborn vaccine trials, especially in the context of ethical standards in medical research. The ongoing scrutiny raises vital questions about how public health interventions are managed, especially in regions that are often the focus of international studies. As the dialogue around vaccination and its ethical boundaries expands, so too does the imperative for responsible and equitable health solutions globally.
Concerns Surrounding the Guinea-Bissau Vaccine Trial
The planned guinea-bissau vaccine trial has raised significant ethical concerns, particularly regarding the administration of the hepatitis B vaccine to newborns. The World Health Organization (WHO) has labeled the study unethical, emphasizing that administering a life-saving intervention to some infants while withholding it from others could lead to irreversible harm. Given the vaccine’s proven effectiveness in preventing the transmission of hepatitis B from mother to child, the WHO advocates for immediate vaccination of all newborns within 24 hours of birth. The organization insists that delaying administration puts vulnerable populations at risk, especially in a region with high hepatitis B prevalence.
Moreover, critics of the study, including medical experts and former health officials, argue that such trials should be conducted within ethical frameworks that prioritize children’s safety and well-being. The WHO’s objections are rooted in a broader concern for ethical research practices, highlighting that established interventions should not be treated as experimental in populations that already face significant health burdens. The ongoing debate underscores the delicate balance between advancing medical research and ensuring the ethical treatment of vulnerable groups in global health settings.
The Role of WHO in Ethical Vaccine Trials
The World Health Organization plays a pivotal role in ensuring that vaccine trials adhere to ethical standards, particularly in low-resource settings like Guinea-Bissau. By questioning the scientific justification for the US-funded hepatitis B vaccine trial, the WHO emphasizes the need for robust ethical safeguards. The organization argues that ethical research must prioritize the welfare of participants, particularly in studies involving children where the risks and benefits must be thoroughly evaluated. The WHO has continually stated that trials which entail withholding an established, life-saving vaccine, such as the hepatitis B birth-dose vaccine, are not justifiable when effective treatments are readily available.
Compounding these ethical considerations is the global context of vaccination practices. With the hepatitis B vaccine having a history of successful use across over 115 countries, the WHO maintains that delaying vaccination for the purposes of trials undermines public health efforts. The agency firmly believes that protecting newborns from a disease that can lead to chronic infection and severe health complications is paramount, thus supporting initiatives that promote timely vaccination as the standard of care. This commitment to ethical research continues to guide discussions on vaccine efficacy and safety across the globe.
Hepatitis B and Its Impact on Global Health Initiatives
Globally, hepatitis B remains a public health concern that underscores the importance of vaccination as a preventive measure. The WHO highlights that approximately 12% of Guinea-Bissau’s adult population suffers from chronic hepatitis B infection. Newborn vaccination is not only a crucial step in preventing the transmission of the virus from mother to child but also a vital intervention in curbing the disease’s prevalence in future generations. By ensuring that every newborn receives the hepatitis B vaccine at birth, countries can significantly reduce the incidence of chronic infections and the associated health complications, such as cirrhosis and liver cancer.
In many regions, including sub-Saharan Africa, addressing the hepatitis B epidemic requires a multi-faceted approach that includes not only vaccination but also public health education and access to healthcare services. Initiatives like the WHO’s endorsement of the birth-dose hepatitis B vaccine aim to bridge the gap in healthcare access for the most vulnerable populations. By prioritizing timely vaccination and raising awareness about the importance of preventing hepatitis B infection, public health officials can foster a healthier future for all, effectively combating this silent epidemic.
Public Health Implications of Vaccine Trials in Guinea-Bissau
Vaccine trials in developing nations bring forth pressing public health implications, particularly when they involve vulnerable populations like newborns. The case of the US-funded guinea-bissau vaccine trial emphasizes the potential to exacerbate health disparities rather than alleviate them. With significant portions of the population affected by hepatitis B, any delay in vaccination poses serious public health risks. The WHO’s recommendation for immediate vaccination highlights the critical role of public health interventions in safeguarding the well-being of infants in high-risk scenarios.
Furthermore, public perception and trust in vaccination programs are adversely affected by the ethical controversies surrounding such trials. The backlash against the guinea-bissau vaccine trial has demonstrated how quickly public sentiment can shift when ethical principles appear to be compromised. As misinformation regarding vaccines continues to circulate, prioritizing transparency and ethical research practices is vital for rebuilding trust among communities. Effective public health communication is essential, reinforcing that vaccines are not merely experimental tools but proven interventions designed to protect lives.
The Future of Vaccination Programs in Guinea-Bissau
Looking ahead, the vaccination landscape in Guinea-Bissau is evolving as health authorities work toward implementing comprehensive vaccination programs. The planned transition to administering the hepatitis B vaccine at birth is a critical milestone that aligns with WHO recommendations. This initiative could significantly reduce the burden of hepatitis B in the population while reinforcing the importance of timely vaccinations to prevent serious health outcomes. With WHO’s support, authorities can effectively manage the ongoing public health crisis presented by hepatitis B and ensure that infants are protected from day one.
Moreover, as Guinea-Bissau continues to strengthen its healthcare infrastructure, collaboration with international health organizations will be key. By fostering partnerships with entities such as the WHO, the country can enhance its public health response to infectious diseases, ultimately improving health outcomes for its population. Engagement in global health initiatives will facilitate access to resources and best practices, providing a framework for effective vaccination programs that not only target hepatitis B but address other pressing health concerns as well.
Understanding Vaccine Hesitancy Amidst Ethical Concerns
Vaccine hesitancy presents a significant challenge in the global fight against preventable diseases, with ethical concerns exacerbating public anxiety, particularly in regions like Guinea-Bissau. Questions regarding the ethics of vaccine trials, specifically the recently halted guinea-bissau vaccine trial, have raised alarms among communities already skeptical of vaccine efficacy and safety. This hesitancy is often rooted in cultural beliefs, past experiences, and current concerns about the motives behind foreign-funded research. Addressing these issues through community engagement is essential to building trust and encouraging vaccination.
Public health campaigns must prioritize education that emphasizes both the scientific and ethical aspects of vaccination. By working collaboratively with local leaders and health workers who understand the cultural context, health organizations can dispel myths and reinforce the importance of vaccinations as effective public health interventions. Ensuring that communities are informed about the benefits of the hepatitis B vaccine, especially in preventing serious health complications, is critical in overcoming doubts and fostering acceptance among hesitant populations.
Ethical Safeguards in Newborn Vaccine Trials
Conducting vaccine trials involving newborns necessitates strict ethical safeguards to protect this vulnerable group. Ethical frameworks must prioritize the well-being of infants while ensuring that any research conducted benefits public health. The controversy surrounding the guinea-bissau vaccine trial illustrates the necessity of adherence to established ethical standards, particularly the principle of non-maleficence—doing no harm. As the WHO indicated, withholding a proven vaccine from any group of newborns contradicts this principle, raising serious moral questions about the intention behind the study.
In future trials, it is crucial to implement rigorous oversight mechanisms that include ethical review boards composed of diverse stakeholders, including local representatives. This inclusion fosters transparency and helps to ensure that the rights and safety of participants are maintained. By prioritizing ethical research practices, the global health community can build a foundation of trust that allows for the advancement of scientific knowledge while ensuring that vulnerable populations are treated with dignity and respect.
The Importance of Global Health Partnerships
Strengthening global health partnerships is essential for tackling issues related to vaccine distribution and ethical standards in research. The response to the guinea-bissau vaccine trial highlights the need for collaboration between international health entities and local governments. By working together, these partnerships can facilitate improved vaccine delivery, enhance public health responses, and ensure that ethical guidelines are upheld in clinical trials. Additionally, partnerships can help align national vaccination strategies with global health initiatives, ultimately creating a more robust framework for disease prevention.
Through collaborative efforts, countries like Guinea-Bissau can leverage international resources and expertise in order to build sustainable healthcare systems. This cooperation not only addresses immediate public health needs but also prepares nations to respond effectively to future health challenges. Public health interventions, particularly vaccination programs, become more effective when backed by a united front of global support and understanding, ensuring that every infant has access to life-saving vaccines regardless of their background or location.
Addressing Misconceptions About Vaccines
Misinformation and misconceptions surrounding vaccines remain prominent barriers to public health initiatives, particularly in low-income countries. The backlash against the guinea-bissau vaccine trial serves as a reminder of the consequences that arise from a lack of accurate information. Myths and doubts about vaccines can spread quickly, often undermining efforts to stabilize immunization rates and protect populations from preventable diseases like hepatitis B. Public health messages must combat these misconceptions through clear, accessible communication that focuses on the benefits and safety of vaccines.
Health authorities need to deploy strategies that engage with communities directly, providing factual information about vaccines and confronting prevalent myths. Educational initiatives, alongside community outreach programs, can empower individuals with the knowledge necessary to make informed decisions about their health and the health of their children. By ensuring that communities receive truthful, science-based information regarding vaccines, public health organizations can work towards reducing vaccine hesitancy and promoting higher vaccination uptake across populations.
Frequently Asked Questions
What are the ethical concerns regarding the Guinea-Bissau vaccine trial?
The Guinea-Bissau vaccine trial faced significant ethical concerns from the World Health Organization (WHO), which described the study as unethical. The WHO highlighted that administering the hepatitis B vaccine—a proven effective intervention—only to some newborns would pose risks of irreversible harm, as it could lead to higher rates of hepatitis B transmission.
Why was the Guinea-Bissau vaccine trial for hepatitis B halted?
The Guinea-Bissau vaccine trial was halted primarily due to public backlash and condemnation from the WHO, which questioned the scientific justification and ethical safeguards of the study. The WHO emphasized that all newborns should receive the hepatitis B vaccine promptly, as delaying vaccination could jeopardize health outcomes.
How does the Guinea-Bissau vaccine trial affect public health in the region?
The Guinea-Bissau vaccine trial aimed to investigate health implications related to hepatitis B vaccination, which is critical for public health in a country with high hepatitis B infection rates. However, ethical concerns about trial conditions could undermine public trust in vaccine initiatives and jeopardize overall vaccination efforts in the region.
What is the WHO’s stance on the hepatitis B vaccine for newborns in Guinea-Bissau?
The WHO strongly recommends that all newborns in Guinea-Bissau receive the hepatitis B vaccine within 24 hours of birth, citing its effectiveness in preventing mother-to-child transmission. The organization criticized the trial for possibly depriving infants of this life-saving intervention.
What implications does the Guinea-Bissau vaccine trial have for future vaccine studies involving newborns?
The Guinea-Bissau vaccine trial raises critical questions about the ethical conduct of vaccine studies involving vulnerable populations like newborns. The WHO’s concerns indicate that trials must ensure that proven treatments are available to all participants, emphasizing the need for ethical standards in subsequent newborn vaccine trials.
What are the potential health risks for newborns involved in the Guinea-Bissau vaccine trial?
Newborns participating in the Guinea-Bissau vaccine trial faced potential health risks due to the delayed administration of the hepatitis B vaccine. The WHO reported that delaying vaccination could increase the risk of hepatitis B infections, which can lead to long-term health complications for infants.
What was the purpose of the US-funded vaccine study in Guinea-Bissau?
The purpose of the US-funded vaccine study in Guinea-Bissau was to assess the broader health implications of hepatitis B vaccination in newborns. However, the ethical shortcomings raised by the WHO led to significant controversy and ultimately the suspension of the trial.
How does the hepatitis B vaccine contribute to public health interventions in Guinea-Bissau?
The hepatitis B vaccine is a crucial public health intervention in Guinea-Bissau, where a significant portion of the population is infected. By vaccinating newborns soon after birth, the vaccine can prevent the transmission of hepatitis B, which is vital for controlling outbreaks and improving long-term health outcomes in the community.
What is the timeline for the introduction of the hepatitis B birth dose in Guinea-Bissau?
Authorities in Guinea-Bissau plan to introduce the hepatitis B birth dose nationwide by 2028, aiming to align with global health standards recommended by the WHO. This initiative highlights the country’s commitment to improving vaccination coverage for newborns.
Who expressed opposition to the Guinea-Bissau vaccine trial and why?
Opposition to the Guinea-Bissau vaccine trial was voiced by various figures, including the former health minister Magda Robalo, who argued that the ethical considerations were unacceptable. Public sentiment against the trial reflected concerns about its fairness and potential exploitation of vulnerable populations.
| Key Points | Details |
|---|---|
| Trial Overview | A US-funded hepatitis B vaccine trial involving newborns in Guinea-Bissau was halted due to ethical concerns. |
| WHO’s Position | The World Health Organization criticized the trial as unethical and emphasized the importance of the birth-dose vaccine. |
| Ethical Concerns | The WHO raised issues regarding scientific justification, ethical safeguards, and standards for human research. |
| Vaccine Efficacy | The birth-dose vaccine effectively prevents 70-95% of mother-to-child transmission of hepatitis B. |
| Population Impact | About 14,000 infants were set to participate, with significant rates of chronic hepatitis B in the population. |
| Current Protocol | Currently, the vaccine is administered at six weeks, with plans to introduce a birth dose by 2028. |
| Community Response | The Guinea-Bissau government suspended the trial after public outcry and criticism from health professionals. |
Summary
The Guinea-Bissau vaccine trial has faced significant backlash and condemnation from the World Health Organization, emphasizing ethical issues and the established efficacy of the hepatitis B birth-dose vaccine. The proposed trial’s cessation highlights the importance of adhering to ethical standards in health interventions, especially when dealing with vulnerable populations such as newborns. With ongoing efforts to protect infants from hepatitis B, it is crucial that health policies align with proven medical practices to ensure the well-being of the population.